Description
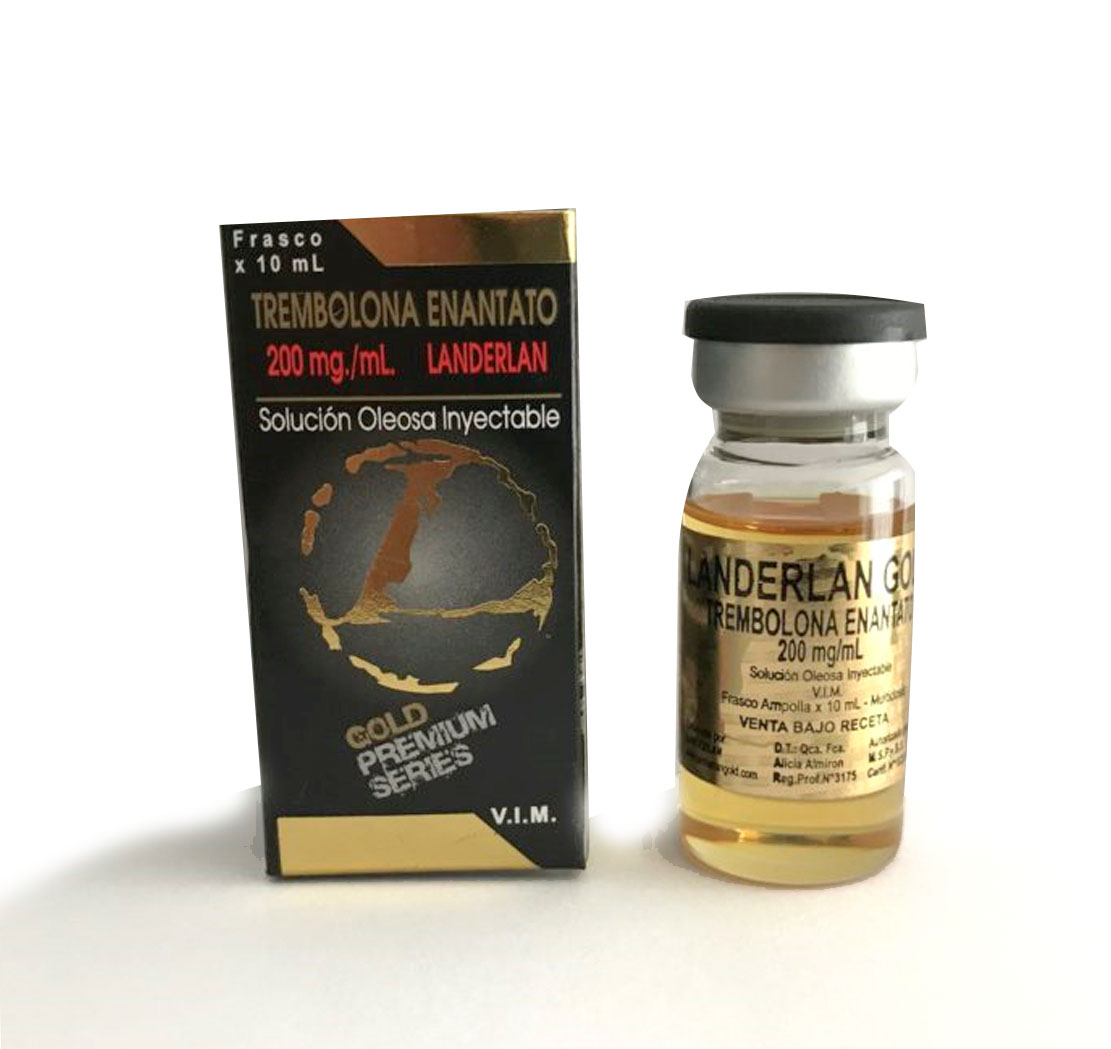
Trenbolone Enanthate Landerlan
INJECTABLE OIL SOLUTION INTRAMUSCULAR
FORMULA
Every 1ml. of Injectable Solution contains:
Trenbolone enanthate.……………………..200 mg x 10ml
Excipients………………………………….cs.
What is Trenbolone? It is a medicine that contains Trenbolone Enanthate which belongs to the Androgen group. Trenbolone is basically in charge of developing and maintaining the body in recovery from debilitating catabolic processes and helping several systems, including the muscular system.
How does this medicine work? It works by activating its highly selective anti-catabolic properties, helping to maintain protein levels in the body, increasing erythropoiesis and increasing the state of conservation and development of the muscular system, as well as regulating fat.
Who should be careful to use this medicine?-Those people with heart disease, high blood pressure, tumors, breast and prostate cancer, prostatic hyperplasia, behavioral disorders, liver problems, poor kidney function, unbalanced calcium in the blood, thyroid, acne, excessively oily skin, severe or chronic respiratory conditions. Especially in women, due to its hormonal action, it is prohibited and contraindicated. Also children, adolescents and elderly people. Pregnant and lactating as it is potentially risky. It should not be administered to those who receive other immuno-weakening treatments, they should necessarily consult their doctor.
THERAPEUTIC ACTION
For the treatment of diseases and conditions that require an increase in protein synthesis.
POSOLOGY
Recommended dose in men 150 mg per week, or according to medical criteria.
More information about Trenbolone




Reviews
There are no reviews yet.